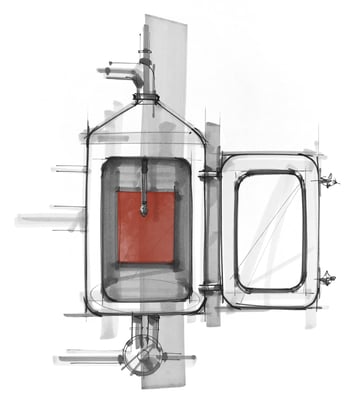
5 Reasons Why Critical Cleaning Chemicals Are So Important To Cleaning Validation
In the high-stakes pharmaceutical manufacturing industry, numerous hurdles must be overcome before successfully bringing a product to market. The “drawing board” may be where new products gestate, but when it comes to bringing ideas to fruition, it all begins — and ends — with cleaning validation. The purity and integrity of a given product is paramount. Cleaning validation is intended to ensure that the product contains nothing more, and nothing less, than what is intended. Your success, reputation, and FDA approval all depend on it. Here are five reasons why applying the right critical cleaning chemicals is essential to cleaning validation:
-
Regulatory requirements for validation
-
To prevent microbial or cross-contamination
-
To ensure operator, environmental, and equipment safety
-
Downtime reduction
-
Maximum equipment utilization (equipment longevity)
Elaborating on these points, we can conclude that knowledge of cleaning helps in identifying the right detergent for the product residues, satisfying all GMP, regulatory and profit requirements of a company.
How to tell if your using the wrong detergent >
1. REGULATORY REQUIREMENT OF VALIDATION:
Pharmaceutical cleaning should be consistent, robust, and repeatable. In case of availability of WIP or CIP systems, their utilization will result in a fixed recipe that can be documented and will not change with change in personnel. However, when a wrong detergent is used, the cleaning time and process usually are not robust and change with the change in personnel. At times, re-cleaning might be required, which is not ideal in a validated process.
2. PREVENTION OF MICROBIAL OR CROSS-CONTAMINATION:
With the wrong process cleaning detergent, it is possible that a residue might remain behind after cleaning, unnoticed visually or analytically. This might happen if:
- The residue is of an excipient, not the API, and thus it is not identified during analytical testing. The residue might be visually undetectable due to its position (difficult to reach/see equipment parts) or due to its transparency.
-
- Depending on the residue itself, this could serve as a potential substrate for microbial growth.
- At times, the API could be embedded in this residue, resulting in potential cross-contamination after a product changeover, though the cleaning has been previously validated.
- The residue precipitates or settles easily from the cleaning agent resulting in re-deposition of the contaminants on other parts of the machine. This could occur randomly and is not easily identified during the cleaning validation.
- Wrong detergents could lead to the necessity of manually scrubbing the equipment, which could further lead to scratches on the contact parts. It has been seen that over time, these scratches tend to accumulate the residue and cause microbial and/or cross-contamination.
Avoidance of Contamination Is the Goal
For obvious reasons, pharmaceutical manufacturing is necessarily subjected to the most stringent quality control standards imaginable. These concerns also come into play in industries involved in producing products intended for intimate human use, such as nutraceuticals, personal care products (e.g., sunscreens, lotions, and cosmetics), etc.
Manufacturing equipment, including flexible machines such as mixers and homogenizers, are capable of being adapted for many processes and products. Switching between different processes (and substances) obviously warrants careful cleaning to prevent contamination of any subsequent products. For example, sunscreens and lotions are notoriously difficult to clean, as these products tend to leave behind persistent residues. Proteins in the biopharmaceutical industry are another example.
Good Manufacturing Practice (GMP) and Quality Systems Regulations (QSR) mandate that any physical surfaces, including any machinery used in production, must undergo cleaning to guarantee that no undesirable substances can contaminate the product in question. Furthermore, the effectiveness of this cleaning must be proven. That’s where validation comes in.
3. TO ENSURE OPERATOR, ENVIRONMENTAL & EQUIPMENT SAFETY:
The cleaning agent to be used for a product on a piece of equipment must be safe for the operators, should fulfill EHS requirements, and be compatible with the MOC of contact parts of the equipment. Basically, the right detergent means no need to re-clean, and no manual cleaning, which means less exposure to the chemical.
4. DOWNTIME REDUCTION:
The time taken to clean equipment is time lost on productivity. Minimizing the equipment cleaning downtime minimizes the nonproductive activity of the manpower and maximizes the production time, hence the profits, and this could be done by identifying the right detergent and cleaning process. We have saved companies big dollars by cutting their cleaning time, which allows them to produce more with the equipment they have.
5. MAXIMUM EQUIPMENT UTILIZATION:
By identifying the right detergent that reduces downtime, the cost of the equipment could be recovered by increased manufacturing, and the need to purchase additional equipment to meet the production demands is reduced considerably. Savings are realized on capital expenditures and/or production is increased, again helping the bottom line.
Using the right detergent helps increase the machine's life by preventing scratches, the need for passivation, and reducing the frequency of changing the non-stainless steel parts - such as gaskets, seals, filters, etc.
Dober Offers the Products — and Cleaning Process Consultation — You Need
All this attention to complex details can be daunting. The good news is, working with Dober, manufacturers need not navigate these obstacles blindly. We offer the Chematic® line of formulated industrial detergents, along with the necessary know-how of cleaning. Our highly effective detergents are customized for your industry and/or specific process, and our consultants will work with you to devise the right formulation and cleaning processes to help you minimize costly downtime, while maximizing manufacturing productivity.
Together, we can devise a plan — including appropriate cleaning products and processes — to achieve reliable, consistent cleaning validation. Proper cleaning can maximize asset use, boost production capacity, improve operator safety, and reduce cleaning time and contamination risk. Ultimately, that can improve your bottom line.
For more information about how the cleaning experts at Dober can help, contact us today.


.jpg?width=352&name=Chematic_Containers_01%20(1).jpg)