How We Scale Up Your Cleaning Process
As it is true in every operation, scale-up is of utmost importance. Like the scale, this step will magnify everything including cleaning difficulties as well as the complications in the cleaning process. Hence, it is necessary to conduct a scale-up activity for the cleaning process too, keeping into consideration, the final equipment where the processing, thus, the cleaning will take place on a regular basis.
Step 1: Consider the cleaning goals, equipment design, and cleaning capabilities
Our cleaning recommendation begins with Custom Cleaning Evaluation studies which are essentially lab-scale cleaning trials, where we evaluate the best detergent and cleaning procedure customized for the final application. Therefore, our CCE (Custom Cleaning Evaluation) form includes questions such as the goal of the cleaning study and the cleaning capabilities available to the particular machine with cleaning issues.
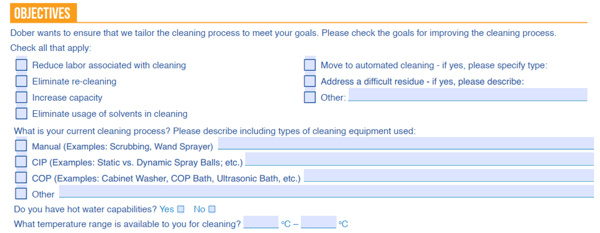
Without this information, the cleaning recommendation provided may not be suitable for the end equipment.
For example, to clean up pigmented HPMC-based film coating, the right detergent would be Chematic® 422. However, the optimized cleaning procedure recommendation will depend on whether the machine is cleaned manually, using semi-automated or automated cleaning. Lower concentrations and temperatures could be used for manual cleaning but as we move to an automated cleaning process, the detergent spray pressure will determine the other TACT parameters. Lower temperatures cannot be used due to possible foaming complications and in the absence of high-pressure detergent spray, we may have to recommend higher detergent concentrations. At times, some highly efficient detergents are recommended only if the cleaning is fully automated and we would provide an alternate cleaner recommendation for the same residue for manual cleaning applications keeping in mind the safety of operators.
Step 2: Scale up in lab scale to check the robustness of the cleaning process
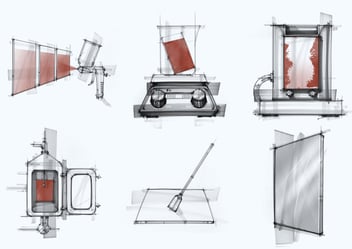
Once we conduct lab-scale CCE trials in the form of Beaker studies, we move up to scaling up devices like the laminar flow, which are available at our Dober facility. At times we have also conducted cleaning trials on pilot-scale machinery such as the coating pan and fluid bed equipment.

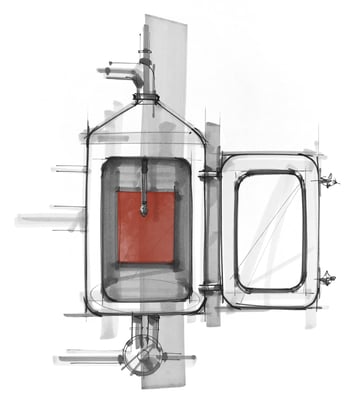
The laminar flow machine with recirculation and temperature maintaining capabilities mimics recirculated CIP cleaning of walls of a large equipment (tanks, FBE, processors, etc) through one or multiple spray balls at a low to medium pressure, and the detergents wetting & cleaning the residue by gravity flow. While the action of the detergent is still minimal, it is slightly higher than the action in the beaker studies and helps to assess if increased action improves residue removal.
Step 3: Scale up in pilot scale to assess the impact of increased action and for process optimization
Once the right cleaning chemistry is determined and confirmed, optimization is the next step for scale-up. It is often observed that there is higher action in both, manual cleaning (where scrubbing is employed) and automated cleaning with pressure spray from the static and/or dynamic spray ball, and this should allow for a decrease in detergent concentration and/or other parameters.

The Pilot CIP system at Dober is a complete small-scale CIP unit with detergent recirculation and reheating capability and variable spray pressure. A large, soiled coupon can be placed in different positions with respect to the spray balls to vary test conditions. This unit helps us to offer optimized cleaning procedure recommendations to our customers and at the same time predicts detergent behavior with high-pressure spray at the temperature range available in the customer CIP cleaning system. It may also foresee effect of scale-up on other parameters such as detergent contact time and ease in removal of insolubles with the increased action.
Scaling up for purely manual cleaning is paradoxically easy and tough at the same time. On one hand, the cleaning action asks for lower detergent concentrations and cleaning process robustness, while on the other, it is difficult to clean a large-scale machine effectively and efficiently with manual cleaning and it is totally operator-dependent.
Step 4: Cleaning Process optimization on actual manufacturing equipment
Although the scaling-up steps give an indication of how the final cleaning on the actual processing machine would turn out to be, a final cleaning process optimization step is always needed due to the large number of variables, like product residue and dirty hold time. In the absence of steps 1-3, this process would be tedious and would need large detergent amounts as multiple optimizations would be required for manual or automated cleaning.
On several occasions, we have seen that without a systematic scale-up approach for a cleaning process development, the final cleaning is less robust and prone to failures, requiring recleaning. It is very important to consider cleaning as a process in itself and develop it during product development, scaling it us as the new product is scaled up.
Conclusion
For products that are already being manufactured regularly, like in the ‘Chicken or Egg conundrum’, Pharma companies need a validated method to determine the new cleaner residue before using it in the GMP machine and would be hesitant to perform this method validation without being sure if the cleaner is worth the effort. We help these companies with our scale-up capabilities to provide a certain degree of assurance that Chematic® detergents deliver what it promises and simultaneously, saving time & effort that they would otherwise need to put into a complete scale-up and optimization.