Cleaning Validation Support
Cleaning validation is made easier with Chematic's Analytical Tech Transfer Package.

Add To Your Analytical Team During Validation
Cleaning validation can place time constraints on your analytical resources. With Chematic®, you will be provided with everything needed to achieve validation for our formulated detergents. We provide an entire analytical technical transfer package with all documentation including:
• Toxicology Data (PDE/ADE Values)
• Chemical & Physical Data
• Validated HPLC & TOC Test Methods
• Transfer of Analytical Procedures
• Technical & Validation Support
*with approved NDA/CDA
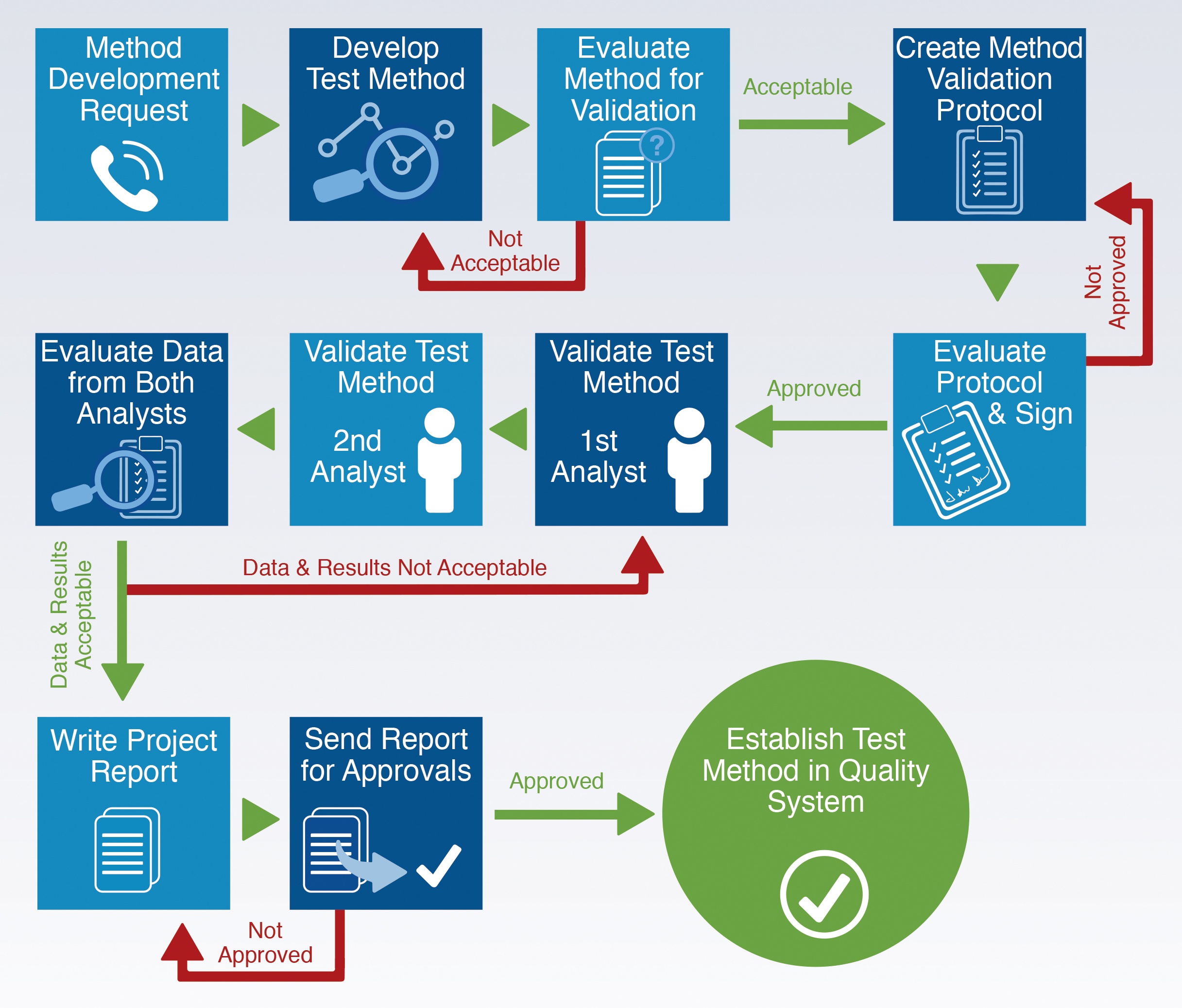
How Does It Work?
Analytical method validation is the process used to prove that the analytical procedure established for a specific test is suitable for its intended use. Chematic's test method validation process is comprehensive, consistent with current pharmaceutical regulatory guidelines and cGMP, fully documented, and ensures that the test results will be reliable and fit for its purpose.