How do Natural Polymers Compare With Synthetics for Wastewater Treatment?
Coagulation and flocculation are the most widely used approaches for solid and color removal from wastewater.
The conventional methods of solid-liquid separation — such as filtration, sedimentation, centrifugation and flotation — are not efficient enough to completely remove suspended particles from wastewater.
The destabilizing effect of coagulation and flocculation processes facilitates the solid-liquid separation, leading to clearer effluents.
Download Your Copy of Our Water Treatment Product Catalog
what are Coagulation and Flocculation?
Coagulation as a process focuses on the neutralization of the surface charge of colloidal impurities (size 0.01-1 μm), converting them into micro-floc. Aggregation of micro-floc to form higher-density, easily-settleable floc is called flocculation.
Coagulation requires the use of very high ionic charge, low molecular weight molecules, that might be inorganic or organic in nature. Flocculation, on the other hand, depends on the use of high molecular weight (greater than 1 million amu) polymers. The long polymer chains of flocculant molecules allow bonding or bridging between micro-floc to take place. The ultimate result is larger floc with higher density and faster settling characteristics.
The choice of coagulation and flocculation chemistry depends mainly on the type, concentration and charge of suspended solids.
Under normal conditions, particles suspended in water carry a negative charge and repel each other when they come closer together. These repulsive forces stabilize the suspension and hinder further interparticle coalition, growth and settling. Neutralization of the negative charge through electrostatic attraction requires an initial use of positively charged (cationic) chemicals.
The dosage of the cationic agent needed to achieve utmost destabilization of suspended particles is critical. Once exceeded, stabilization and resuspension of the particles will take place because of the excess positive surface charges.
Direct Flocculation
Wastewater treatment professionals usually apply a sequential process of coagulation and flocculation (C/F) to maximize the formation of larger and denser floc that are easier and faster to settle (Figure 1).
Whenever wastewater is heavily contaminated with organics, a more economical non-coagulation (direct flocculation) approach is followed. In this one-step process, polymer molecules are efficiently adsorbed on the contaminant particles and initiate bridging, floc growth and sedimentation. The higher solid content in this case improves interparticle interaction and minimizes the polymer dosage required for efficient flocculation.
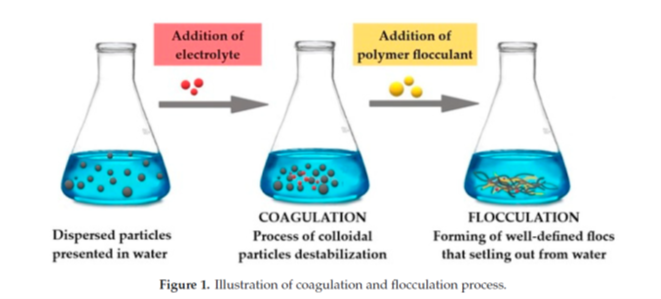
"Illustration of coagulation and flocculation process" by Maćczak et al is licensed under CC BY 4.0.
While the goals of the wastewater treatment process are obvious, the mechanism of the coagulation and flocculation steps is vague and could either depend on charge neutralization, bridging of micro-floc or a combination of both. Several factors, including the contaminant concentration, the dosage, the identity, the structure, the type of charge, the charge density, and the molecular weight of the polyelectrolyte will determine the exact mechanism of each step.
It’s very important to notice that the coagulation process is mainly driven mainly by charge neutralization while the flocculation step is majorly the result of inter-particle bridging. This explains why large molecular weight polymers (1-30 million amu) are more efficient as flocculants and do not really have to carry an opposite charge to micro-floc.
Flocculants could be neutral or carrying the same or opposite charge of the suspended particles. It must be emphasized that both coagulants and flocculants need to be adsorbed on the particle surface upon contact. The adsorption process is an equilibrium process and takes some time to be complete. Metal ions such as calcium ions could play a crucial role in the adsorption of anionic polymers on particles of similar charge despite electrostatic repulsion. In this ion binding scenario, the positively charged Ca 2+ ions act as bridges between the negatively charged particles and the anionic groups on the polymer chains.
Encapsulation Systems
In addition to charge neutralization and inter-particle bridging, an additional encapsulation mechanism could come to play and initiate flocculation. This special case requires the presence of two oppositely charged (one cationic and one anionic) polymers.
The hydrogen bonding or the electrostatic interactions between the oppositely charged polymer molecules create a crosslinked network of polymer chains that can physically entrap or encapsulate the contaminant particles, regardless of their surface charge.
types of Synthetic Polymers
Coagulation and flocculation chemistries are versatile and could use either inorganic or organic (carbon-based) chemicals. Each of these two major categories offers characteristic environmental and economic advantages while promoting solid–liquid separation (dewatering) processes. Carbon-based water treatment polymers could be synthetic, semisynthetic, or natural polymers.
Synthetic polymers are mostly derived from petroleum or natural gas products through polymerization. They include polyacrylates, polyacrylamide, polyamines, poly DADMAACs, and poly (ethylene oxides), etc.
Synthetic Cationic Polymers
Synthetic polymers are available in a wide range of molecular weights and charge densities. They could be cationic, anionic, or nonionic in nature.
Cationic polymers could be used as coagulants or flocculants based on their molecular weight range. As coagulants, cationic polymers of low molecular weight (20,000 to 1 million amu) and high charge density — such as quaternary polyamines, poly DADMAC and dicyandiamide resins — are well known. The coagulant molecules are getting adsorbed on the surface of negatively charged colloidal particles, forming localized regions of positive charges (Patch Model). These partially neutralized particles could act as real dipoles and get attracted to each other as indicated in Figure 3, leading to the formation of micro-floc.
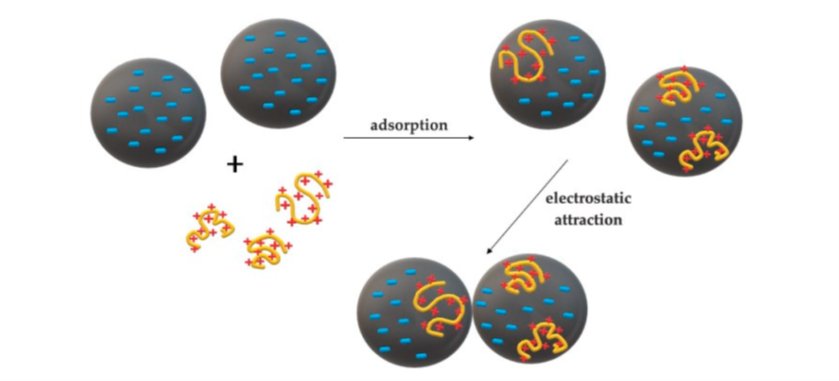 Figure 3, "Mechanism of electrostatic patch model for flocculation (according to Bolto" by Maćczak et al is licensed under CC BY 4.0.
Figure 3, "Mechanism of electrostatic patch model for flocculation (according to Bolto" by Maćczak et al is licensed under CC BY 4.0.
Synthetic cationic polymers offer several advantages as coagulants compared to the tri- and divalent inorganic counter parts that work mainly through the sweep floc mechanism.
First, they neither require strict pH control nor consume alkalinity and, hence, could be used for treating a wider variety of water. Despite their higher cost, the dosage is at least 10 times lower, making them more economic. The shear stability of the floc is much better, resulting in less carryover and a cleaner effluent. In addition, the generated sludge is denser and easier to compress.
As flocculants, cationic polymers of relatively higher molecular weight (3-10 million amu) and varying charge densities (CD < 15 %) are employed. Cationic flocculants are mainly based on copolymers of acrylamide with quaternary ammonium monomers. As a result of their higher molecular weights, cationic flocculants tend to have considerable bridging capabilities.
types of Synthetic Anionic and Nonionic Polymers
Anionic Polymers
The most widely used anionic polymers contain carboxylic acid groups, which are weakly acidic by nature, so their charge densities are pH dependent. Very high molecular weight (10-30 M amu) and medium charge density (10-30%) carboxylic acid polymers derived from PAM/PAA (polyacrylamide/polyacrylic acid) copolymers are extensively employed as flocculating agents in the water treatment industry, among others.
In addition to their excellent flocculation performance, they tend to bind multivalent metal ions and remove them from water.
Nonionic Polymers
Polyacrylamides
Non-ionic polyacrylamides (PAMs) refer to PAMs with a degree of hydrolysis between 1-3%. The partial hydrolysis of the amide group to carboxylate group creates a limited amount of anionic groups. Non-ionic PAMs are not considered one of the polyelectrolytes and their flocculating efficiency depends mainly on the molecular weight.
Generally, the higher the molecular weight, the better the flocculating efficiency of non-ionic PAMs.
Polyethylene oxides (PEO)
These normally have a high molecular weight (2 to 8 million). Although they are non-ionic in nature, they get strongly adsorbed on hydroxylated surfaces such as silica particles through hydrogen bonding. PEO find applications as flocculants for silica and kaolinite rich wastewater.
Natural Polymers
Natural polymers originate from plant or animal sources and include lignin, tannins and polysaccharides.
Semi-synthetic (sometimes called organic) polymers are produced through chemical or biological modification of natural polymers. Natural and organic polymers are currently receiving a lot of attention due to their advantages over conventional synthetic polymers or inorganic agents.
Biodegradability, non-toxicity, ability to undergo different chemical modifications and availability from renewable sources are the major advantages.
Natural Cationic Polymers
Chitosan
Chitosan is the most prominent naturally occurring amine based cationic polymer.
Chitosan is inherently cationic (17% at pH 7), but the cationicity could be substantially improved at acidic pH. Chitosan is considered a weak electrolyte polymer because its positive charge is pH dependent. The commercial product is obtained through careful deacetylation of chitin, a primary component of the exoskeletons of arthropods. Chitosan's major applications include metal ions removal, treatment of food processing waste, removal of natural organic matter (NOM) and decolorization of dyehouse effluents.
Natural non-ionic polymers
Starches, galactomannans (e.g., guar gum), cellulose derivatives, microbial polysaccharides and gelatins are mainly used as flocculants.
They have different chemical and physical properties but could be chemically modified to impart specific chemical or performance advantages.
Grafting PAM, cationic reagents and surfactant molecules onto guar gum and starch has yielded polymers that are quite effective in solid-liquid separation and emulsion.
Interested in learning more from our water treatment experts? Reach out to us.
References:
- Fakhru’l-Razi Ahmadun et al., Journal of Hazardous Materials 170 (2009) 530–551
- Rey and Varsanik, May 5, 1986. DOI: 10.1021/ba-1986-0213.ch007
- Faheem Akhter et al., DOI: 10.17576/jkukm-2021-33(2)-02
- Brian Bolto and John Gregory, Water Research 41 (2007) 2301 – 2324
- Abd El-Shafey I. Ahmed et al., J. Appl. Polym. Sci. 2014, DOI: 10.1002/APP.40458
- R. Gaudreault et al. Colloids and Surfaces A: Physicochem. Eng. Aspects 268 (2005) 131–146
- Piotr Maćczak et al. Materials 2020, 13, 3951; DOI: 10.3390/ma13183951
- Luminita Ghimici and Marieta Nichifor, Journal of Macromolecular Science, Part B: Physics, 48:1 (2009) 106-113
- Kanika Saxena et al., Environmental Technology Reviews, 7:1(2018) 156-176
- Marcin Lemanowicz et al., DOI: dx.doi.org/10.14314/polimery.2016.092




