Wastewater treatment is the process used to achieve water quality goals required to discharge “used” water back to the environment. An industrial wastewater treatment process is utilized to achieve discharge water quality requirements from Clean Water Act at the Federal level, further delegated down to state and sometimes other municipal bodies.

Wastewater treatment concentrates waste materials, transforms them, and/or segregates them for disposal away from water discharging to the environment or publicly-owned treatment works (POTW). This contrasts to the old adage “dilution is the solution to pollution”, which diffuses pollutants into the environment at discharge.
The processes described below are the major techniques and technologies used for wastewater treatment. Wastewater treatment systems vary widely from site to site, with a “treatment train” that is designed to meet the needs of a particular site, facility, or unit process generating waste products during normal operations. Depending on the source of the wastewater and the discharge water quality requirements, the water treatment steps may be utilized in varied sequences and scales. For example, there may be a cursory solids removal step prior to biologic treatment, followed by a more substantial solids removal step.
Biological Treatment amplifies naturally available processes of biodegradation to remove nutrients (like Nitrogen and Phosphorus) and organic molecules, often measured as BOD or COD (Biochemical Oxygen Demand or Chemical Oxygen Demand). Biologic treatment systems cultivate a microbiologic ecosystem to complete microbial digestion of waste to achieve the water treatment goals. To sustain microbes, the treatment system manages various nutrient cycles. In particular, the Nitrogen cycle and Carbon cycle dictate cellular metabolism and growth, while the presence or absence of oxygen in the water defines the types of microbiological species present.
- Aerobic treatment supports a microbiologic ecosystem with oxygen in order to cultivate species of microbes that will most efficiently digest organic molecules and drive BOD removal via nitrification, resulting in carbon dioxide and biomass sludge.
- Anaerobic treatment supports a microbiologic ecosystem without oxygen in order to cultivate species of microbes that will most efficiently digest organic molecules and drive BOD removal via denitrification, resulting in methane-rich biogas and biomass sludge.
- Anoxic treatment is similar to anaerobic, in that there is no oxygen introduced, however there may be some molecular oxygen available as nitrate (NO3-) or nitrite (NO2-). Anoxic treatment is a less common variation on manipulating the Nitrogen cycle to treat water.
There are many examples of biologic treatment systems, in general they are categorized according to the type of media used for growing the microbes.
In an attached growth system, the microbes attach to an inert media (e.g. plastic balls or lava rocks) as a biofilm. The media may be stationary, as in a trickling filter, or suspended in the water column, as in a moving bed bioreactor (MBBR).
In a suspended growth system, microbes are maintained in a suspension in the wastewater being treated. Within the “mixed liquor”, wastewater will contain solids, individual microbes, or clusters (“flocs”) of microbes.
Mechanical Treatment is the process to separate solids from water through physical and mechanical means. As a general rule, it is inexpensive to remove large particles and increases in cost as particle size decreases. In the context of a wastewater treatment system, solids separation may occur in multiple steps to maximize efficiency and optimize treatment costs.
Gravity can help separate solids particles from water, given adequate time and quiescent or laminar flow conditions. Gravity can be utilized for clarification by either settling or floating solids out of the water column, then removing the stratified solids from the water.
Settling behavior of particles is well studied and predictable using Stokes Law. The chart below summarizes the results of an equation, which shows how particle size, density, and water viscosity dictate the settling rate of solids.
|
Diameter of Particle |
Type of Particle |
Settling Time in 1m Water |
|
10mm |
Gravel |
1 second |
|
1mm |
Sand |
10 seconds |
|
100µm |
Fine Sand |
2 minutes |
|
10µm |
Silt, Dust |
2 hours |
|
1µm |
Clay |
8 days |
|
0.1µm |
Colloids |
2 years |
Examples of settling and clarifying equipment in an industrial wastewater context include circular or rectangular clarifiers, above ground settling tanks, lagoons, or lamella clarifiers.
Another way to use gravity for water clarification includes flotation. This allows solids lighter than water (e.g. algae, fats, oils, greases) to coalesce or gather at the top of the water column, allowing those solids to be collected and removed from the water.
Most often, air bubbles are used to enhance the separation. Air (or sometimes a specific gas) is introduced as bubbles of different sizes. The gas bubbles attach to suspended solids or oily contaminants, decreasing their specific gravity, which allows them to float out of the water column, to form a skimmate layer which is separated from the top of the water.
- Dissolved Air Flotation (DAF) systems utilize bubbles sized 30-50 µm
- Induced Air Flotation (IAF) systems utilize bubbles sized 70-150 µm
Filtration is the process of passing suspended material through a porous material in order to capture the solids while allowing water to flow through. Selection of filter media is dictated by the sizes of particles that are to be removed from the water. In a wastewater treatment train with multiple filtration steps, they are sequenced to remove the largest particles first, stepping down to the smallest particle removal.
Common examples of filters include media filters, bag filters, and membranes.
Media filters may be pressurized vessels or gravity fed basins, as in slow sand filtration systems. Filter beds may be comprised of a variety or mixture of granular filter medias and may be of varying depths. The depth of the media bed provides significant filtration capacity and becomes an advantage for increasing efficiency of the filter.
Medias vary widely. Inert filter media, like sand, gravel, or crushed glass, capture particulate solids down to 10-25 µm. Active filter media, like activated carbon or ion exchange resin, capture solids down to 10-25 µm, but are most often integrated to capture specific dissolved contaminants, like petroleum hydrocarbons, PFAS, PFOA, among others. Active media filters are commonly used at the end of a treatment system as a “polishing” step, downstream of other solids removal steps. By sending clear, pre-treated water to an active media bed, the treatment system most efficiently utilizes the specialty media and minimizes operations costs.
Bag filters are pressurized vessels containing a bag made of cloth, felt, or specialty paper. This single-layer of media filtration is often used as a “polish step” for the finest particles, or as a “safety net” downstream of a more substantial solids-removal process. Bag filters styles vary widely and usually rated to remove particles between 1-100µm.
Membrane filters are pressurized vessels containing a highly technical filter cartridge made of specialty plastics or ceramic. The type of membrane filtration is defined by the pore size of the membrane, Microfiltration (0.1µm), Nanofiltration (0.01µm), and Nanofiltration (0.001µm) down to Reverse Osmosis (0.0001µm). Depending on the type of membrane system, the cartridge may be an assembly of tubes made of the special membrane, or the cartridge may be a solid piece of plastic. Either way, the pores in the plastic are so small that they are not visible to the naked eye. Membrane filters are utilized in the most specialized and technical wastewater treatment processes. Many treatment systems integrate chemical treatment techniques as an alternative to integrating membrane filtration.
Chemical Treatment is the process of manipulating water chemistry to convert or remove contaminants. Chemical treatment may target specific chemical reactions, and it can be used to change the properties of water and its contaminants. Chemical treatment is often employed to enhance a natural phenomenon or increase efficiency of solids separation steps.
pH adjustment is the manipulation of the balance of hydrogen and hydroxyl ions in water, which are the two components of a water molecule.
H2O ↔ H+ + OH-
The pH scale is 1-14, where “neutral” is 7, indicating that the hydrogen and hydroxyl ions are balanced. When the pH of a substance is below 7, it is classified as “acidic” due to the abundance of hydrogen ions available. When the pH of a substance is above 7, it is “basic” due to the scarcity of hydrogen ions available.
A related dynamic is Alkalinity, which is water’s capacity to neutralize an acid. It is measured by the concentration of carbonates, bicarbonates, and hydroxide in the water. While alkalinity is rarely adjusted specifically, it is important to understand in water treatment because it creates “buffering capacity”, which is the ability of water to resist a pH change. A water with a high buffering capacity will require a higher dose of acid or base to change the pH, compared to a water with equivalent pH but lower buffering capacity.
Oxidation (or a “redox reaction”) refers to the transfer of electrons between molecules. This is accomplished with the addition of oxygen to water to create hydroxyl groups (-OH) which can be used to drive disinfection, precipitation of some metals, and odor removal. Oxygen may be added through aeration of water with pure oxygen gas (O2) or with ambient atmosphere. It is more common in wastewater treatment to inject an oxygen-containing liquid into water, such as hydrogen peroxide (H2O2) or chlorine bleach (sodium hypochlorite NaOCl).
Precipitation is the process of changing the form of a material from dissolved in water to a solid particle. This process introduces a counterion that reduces the solubility of the dissolved ion, causing a solid particle to form. Precipitation reactions may be used to remove dissolved metals or dissolved minerals by adding specific chemical reactants, using oxidation, pH adjustment, or electrochemical reactions.
Coagulation destabilizes and neutralizes electrostatic charges on the surface of solid particles in water. This process introduces small, but highly charged molecules into the water to destabilize the charges on particles, colloids, or oily materials in suspension. Sometimes the destabilization of particles from a colloidal suspension results “microflocs”, just barely visible to the naked eye.
There are many wastewater treatment applications that require coagulation reactions, such as removing colloidal solids from water, demulsifying oil emulsions (“emulsion breaking”), and in paint detackification. There are also many types of coagulants available to meet those needs.
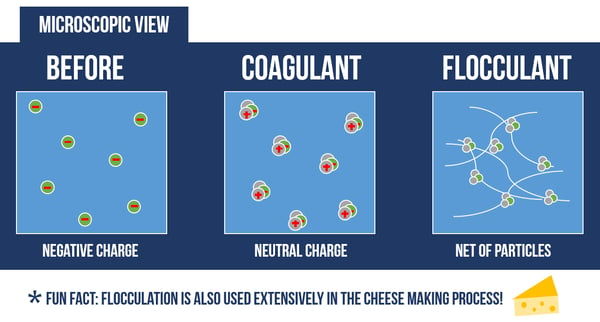
Flocculation is the process of clumping particles together to build larger agglomerates. This process introduces a large molecule with electrostatically charged binding sites to attract oppositely-charged particles or microflocs. The reaction itself is highly visible, as the resulting “flocs” readily separate from the water.
Most wastewater treatment applications integrate some form of flocculation to increase the efficiency of liquid/solid separation processes like clarification or filtration. Most flocculants are a type of polymer, a large chain molecule made of repeating subunits. Polymers used as flocculants may be synthetic (such as Polyacrylamide) or naturally-sourced “biopolymer” (such as chitosan).
Wastewater treatment systems vary from site to site, where techniques and technologies are used in different sequences to meet the discharge water quality requirements of an industrial operation. Wastewater treatment may be a complex process, but there are many resources available to assist in developing and optimizing system performance.
Partner with Dober to help navigate wastewater treatment improvements at your facility.
